Quality and accuracy of early in vitro and in vivo studies occupy an essential role in drug discovery and development processes. While the primary goal of ADME (absorption, distribution, metabolism and excretion) study is to discover high-affinity ligands against the target, monitoring of drug-like properties is also accomplished. The foremost reasons for the failure of many new drug candidates are poor Pharmacokinetic (PK), ADME and Toxicological characteristics. The above factors alone account for up to 50% of the attrition rate of new drug candidates during the drug development process. The high cost of R&D and fast pace with which modern medicinal chemists can synthesize new compounds places a high demand on early ADME/PK groups.
To optimize the pharmacokinetic characteristics of a drug candidate ADME studies are performed. It helps in identification of the metabolic pathways and provides valuable input for the design of in vivo studies. We offer cost-efficient standardized and customized assays as per the client’s requirement for ADME parameters. ADME capabilities of DRIK include the following:
Physicochemical Studies
- Partition coefficient (LogD/LogP)
- Solubility (UV/Visible spectroscopy)
- Chemical stability at different pH
- Biological matrix stability (serum/plasma/blood/microsomal/hepatocytes/tissue homogenates /simulating fluids)
- Reversible and time dependent CYP induction
- 3D Slices
Absorption/Distribution Assays
- PAMPA (parallel artificial membrane permeability assay)
- Caco-2 permeability study
- Tissue permeability studies – Franz Diffusion Assay
Metabolism
- CYP inhibition/Induction (CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4)
- Drug–Drug Interaction (DDI) studies
- Pathway determination (Phase I and Phase II)
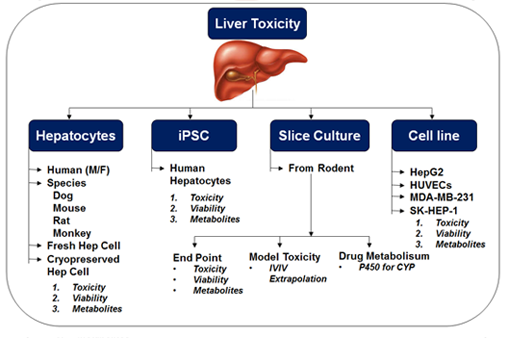
- Half-life/clearance determination using Microsomes / Hepatocytes / S9 fractions / CYP across species (Human/Rat/Mouse/Dog)
- Metabolite identification with Microsomes/Hepatocytes and S9 fraction across species (Rat/Mouse/Dog/Human)
Note:
CYP Inhibitor: Furafylline, Pilocarpine, Quercetin, Fluconazole, Ticlopidine,
CYP Inducer: Omeprazole, Rifampicin, Phenytoin, Quinidine, Ketoconazole
Bio-analysis
We have the capabilities to undertake medium to high throughput Bio-analysis and method development through our partners.